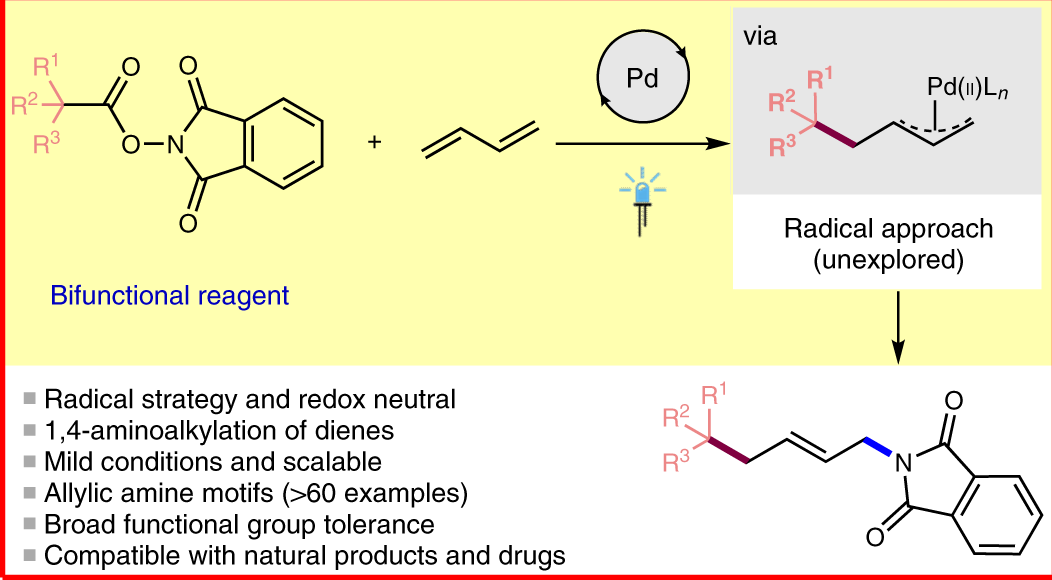
1271-03-0 | Allyl(cyclopentadienyl)palladium | (η5-2,4-Cyclopentadien-1-yl)(η3-2-propen-1-yl)-palladium; (η5-2,4-cyclopentadien-1-yl)(η3-2-propenyl)-palladium; Allylcyclopentadienyl-palladium; π-Allyl-π-cyclopentadienyl-palladium; π ...
![PDF] Computational Insights into Palladium-Mediated Allylic Substitution Reactions | Semantic Scholar PDF] Computational Insights into Palladium-Mediated Allylic Substitution Reactions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b392fe8fdff097ec97ca2e5082d62b74ad9d76a8/12-Figure5-1.png)
PDF] Computational Insights into Palladium-Mediated Allylic Substitution Reactions | Semantic Scholar

Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect
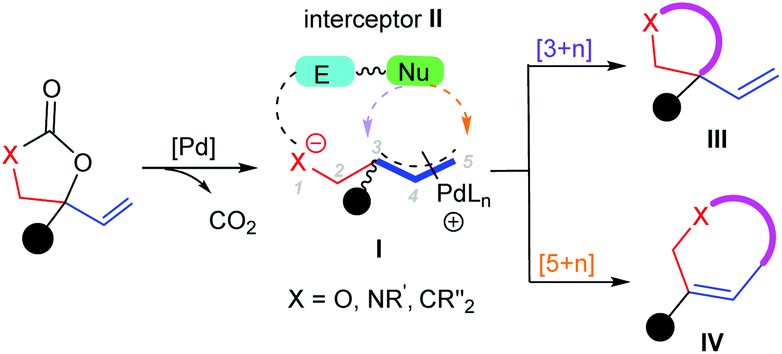
Recent advances in annulation reactions based on zwitterionic π-allyl palladium and propargyl palladium complexes - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00273B

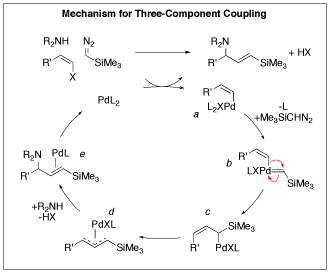
Mechanism of allyl deprotection through catalytic palladium π-allyl... | Download Scientific Diagram

π‐Allyl)Pd Complexes Containing N‐Heterocyclic Carbene and Pseudohalogen Ligands – Synthesis, Reactivity toward Organic Isothiocyanates and Isocyanides, and Their Catalytic Activity in Suzuki–Miyaura Cross‐Couplings - Kim - 2013 - European Journal of ...
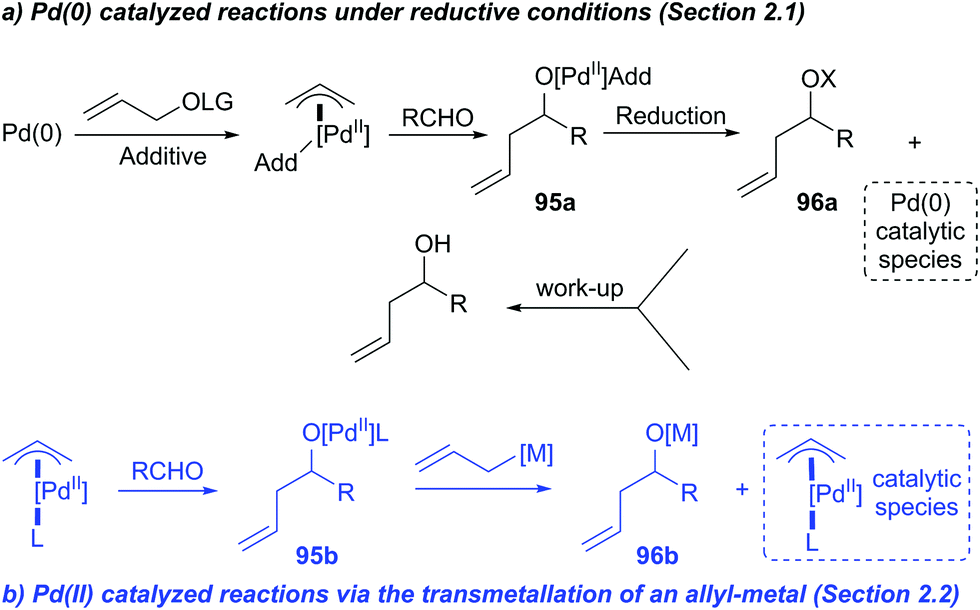
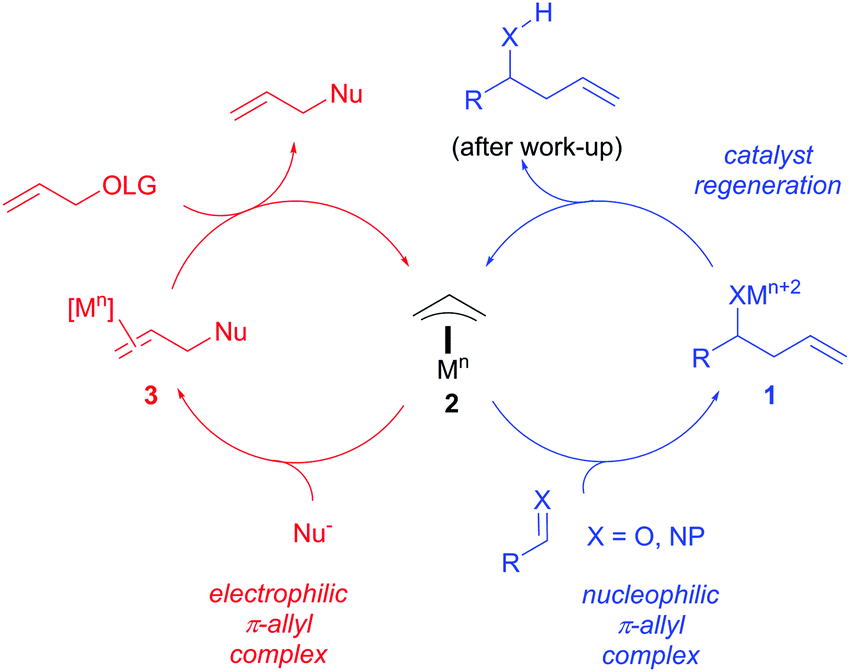
Catalytic nucleophilic 'umpoled' π-allyl reagents - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C7CS00449D
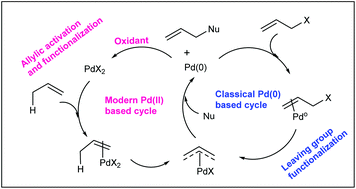
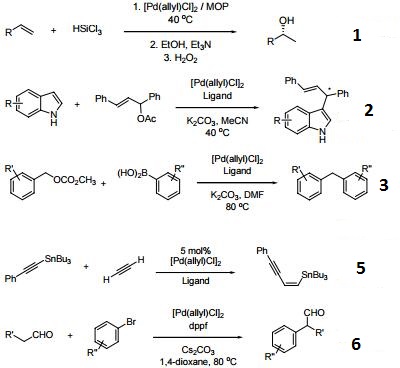
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)
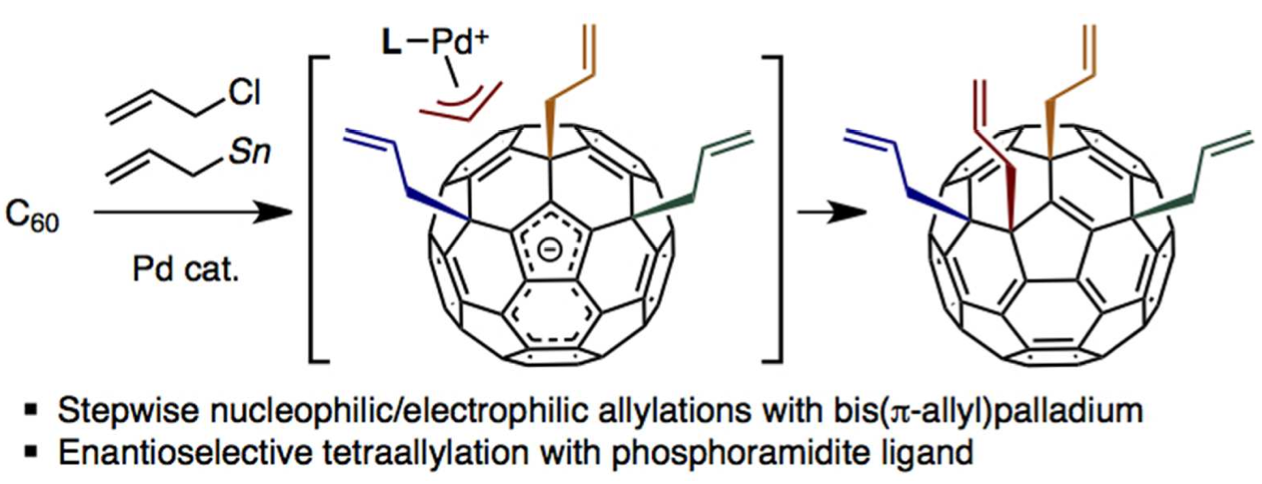
Palladium-catalyzed tetraallylation of C60 with allyl chloride and allylstannane: Mechanism, regioselectivity, and enantioselectivity | Itami Organic Chemistry Laboratory, Nagoya University
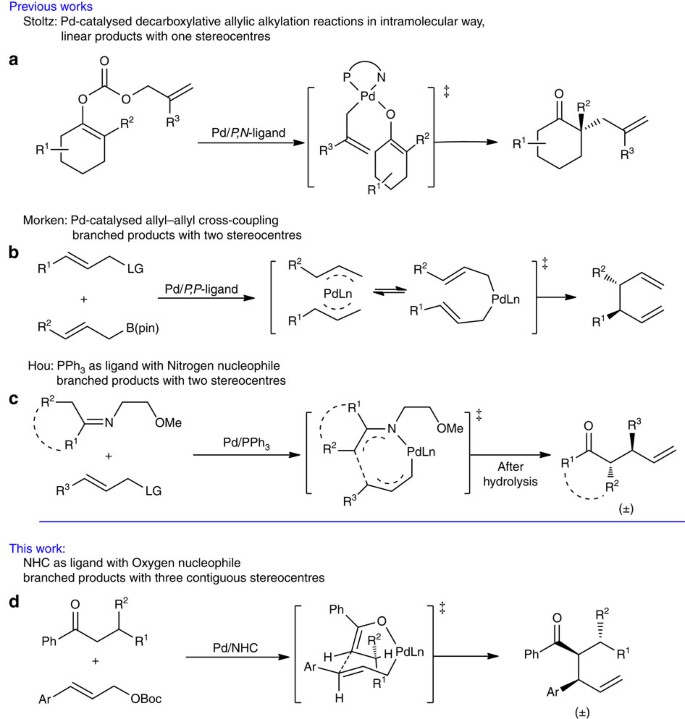
Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications

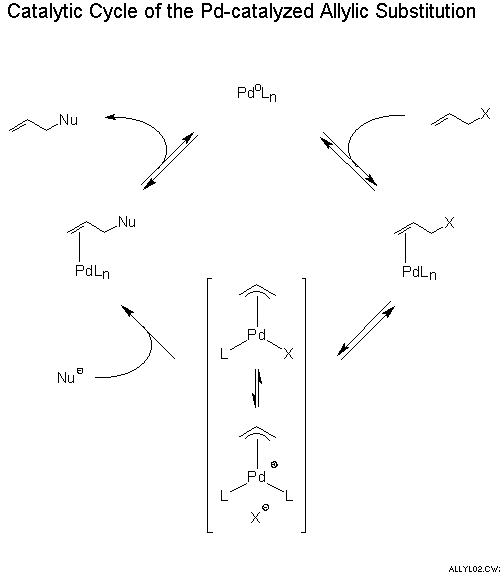
Stereochemistry of the palladium-catalyzed allylic substitution: the syn-anti dichotomy in the formation of (π-allyl)palladium complexes and their equilibration - ScienceDirect

Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization | Nature Communications

Synthesis, characterization, and reactivity of (π-allyl)palladium(II) wrap-around complexes with 1,3-dienes - ScienceDirect

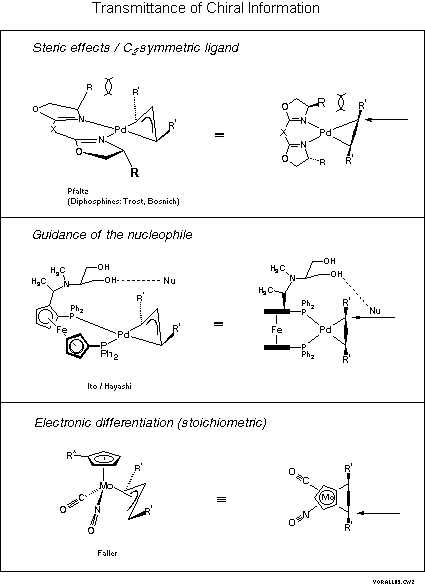
Asymmetric allylic substitution by chiral palladium catalysts: Which is more reactive, major π-allyl Pd(II) species or minor π-allyl species? - ScienceDirect

![Pd(allyl)Cl]2 Umicore | Sigma-Aldrich Pd(allyl)Cl]2 Umicore | Sigma-Aldrich](https://www.sigmaaldrich.com/deepweb/content/dam/sigma-aldrich/structure9/137/mfcd00044874.eps/_jcr_content/renditions/mfcd00044874-medium.png)