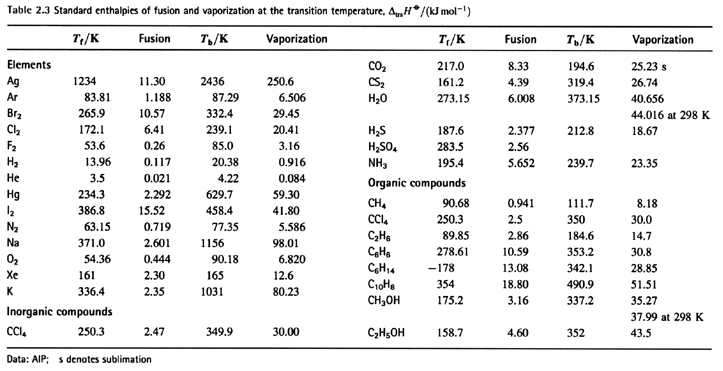
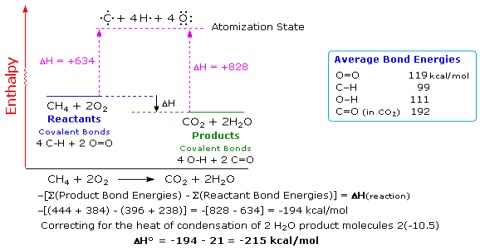
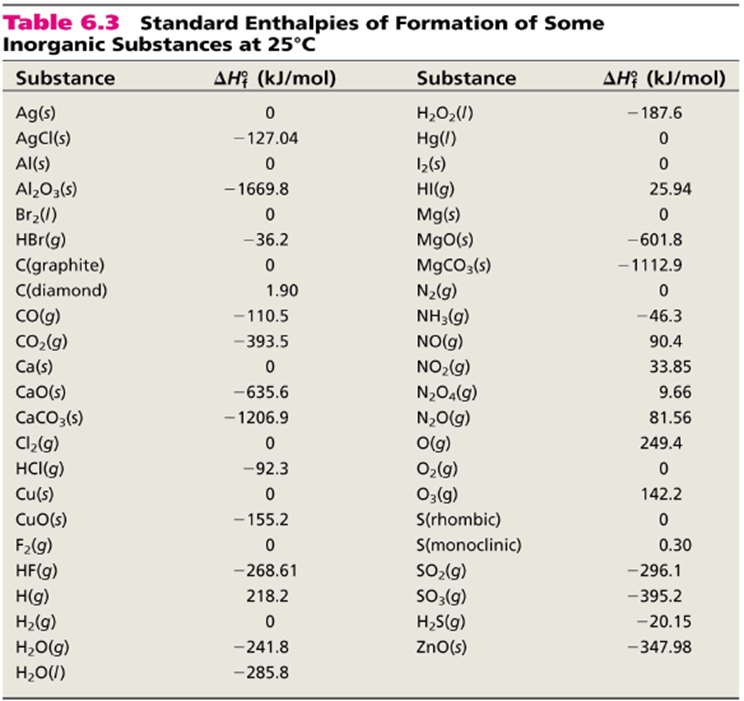
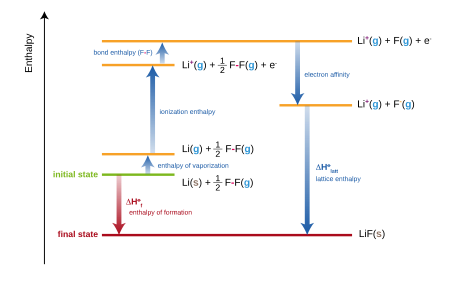
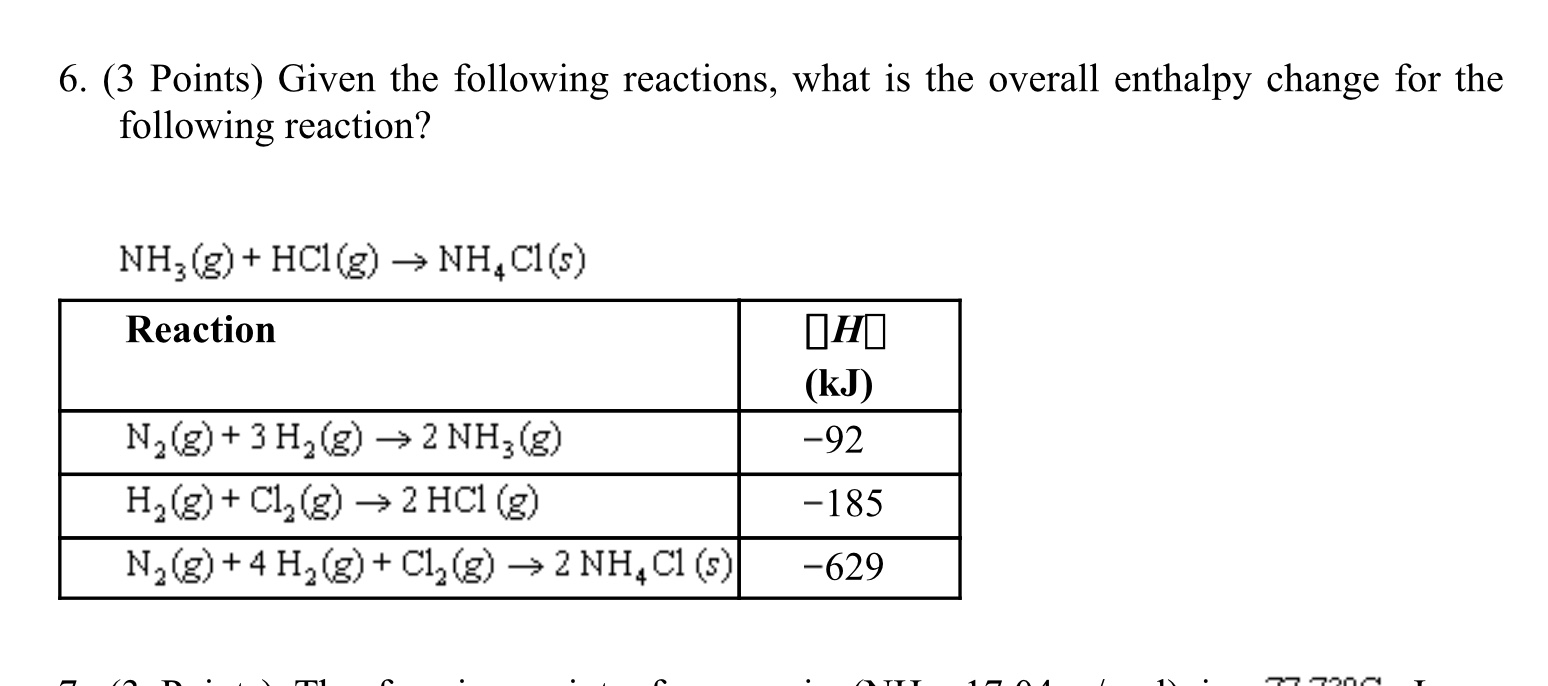
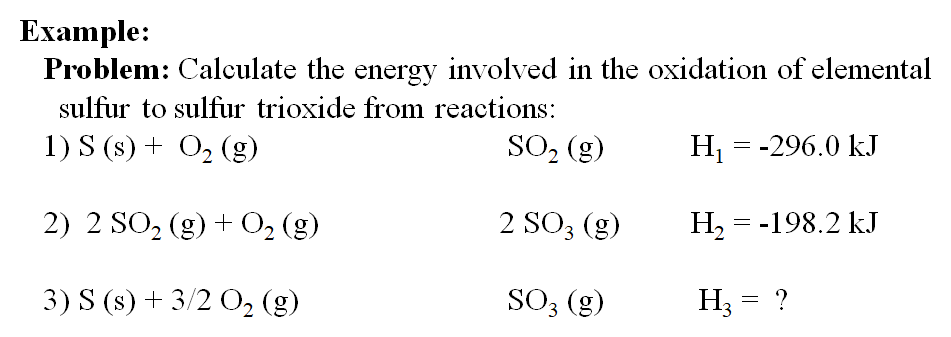SOLVED:6.8 Calculating the overall enthalpy change e of a reaction (Hess's law) (268): AH, oucnii AH, AHz AH, 6.9 Calculating the standard enthalpy of reaction (2ZV): AHO EmAH? Groducts) EnAH?aextats) Answer the

A level A Level 1.1 Advanced Introduction to Enthalpy (Energy) Changes reaction, combustion, formation in Chemical Reactions KS5 GCE chemistry revision notes

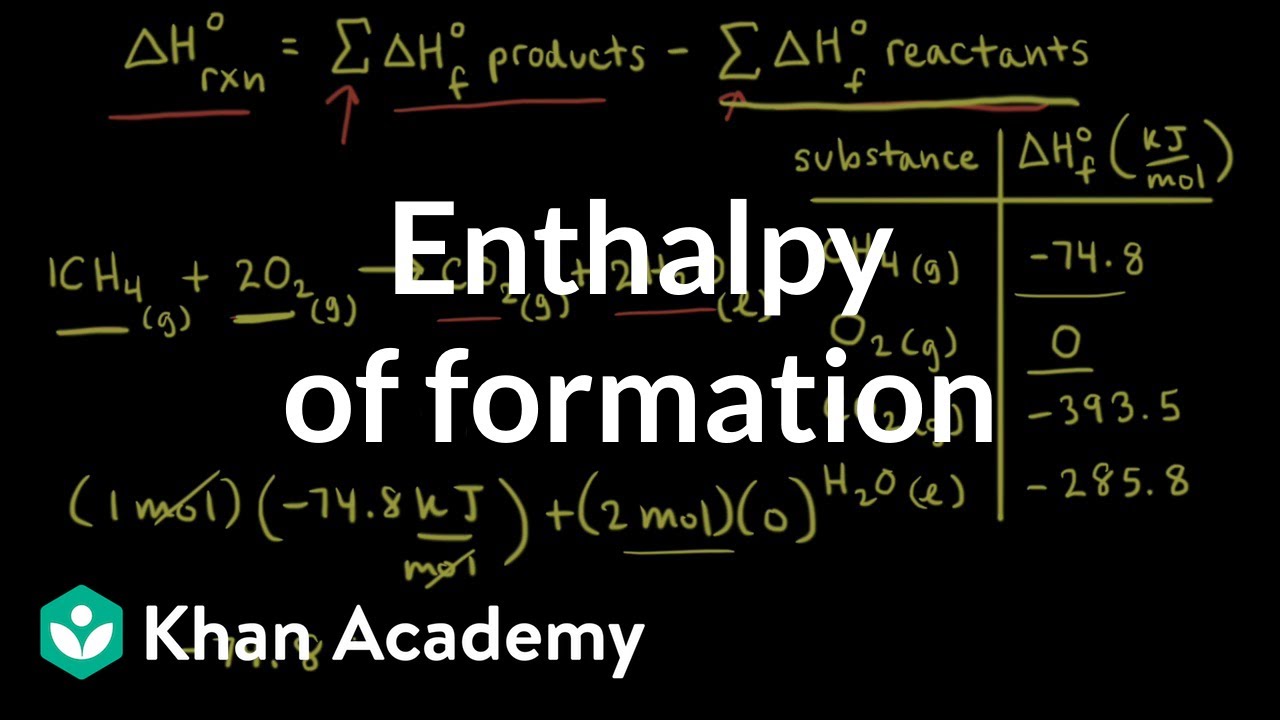
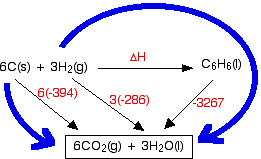
Hess's law states that : ( )A. the standard enthalpy of an overall reaction is the sum of the enthalpy changes in individual reactionsB. enthalpy of formation of a compound is same as the enthalpy of decomposition of the compound into constituent ...
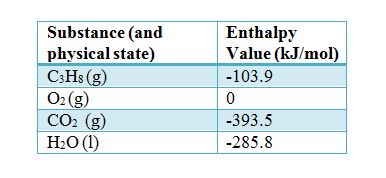
Calculate the enthalpy of reaction for the reaction "CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2? | Socratic