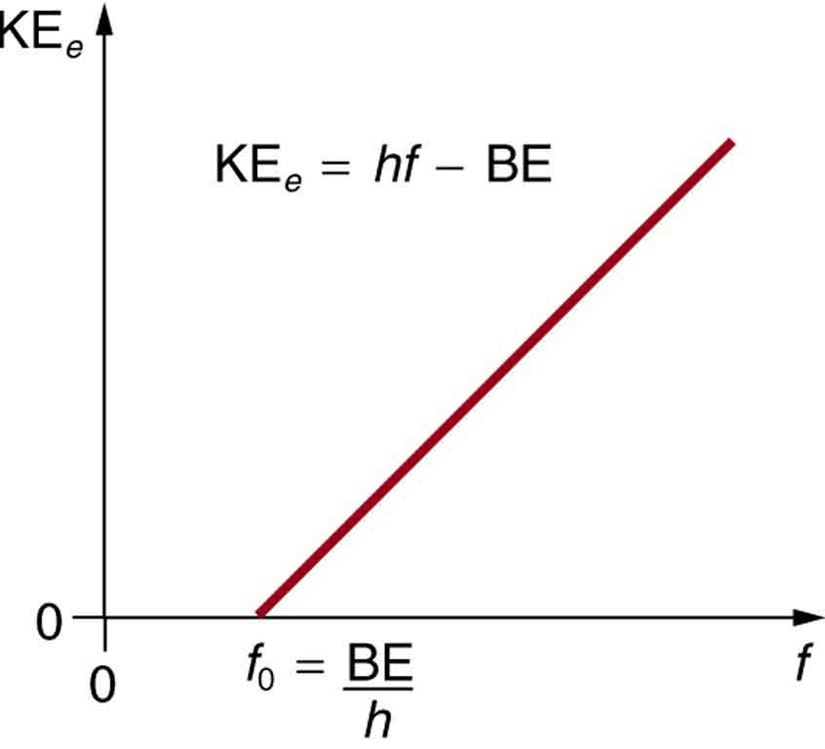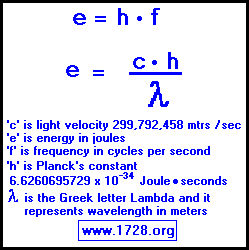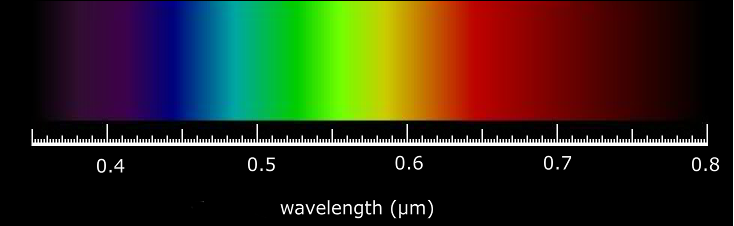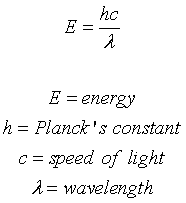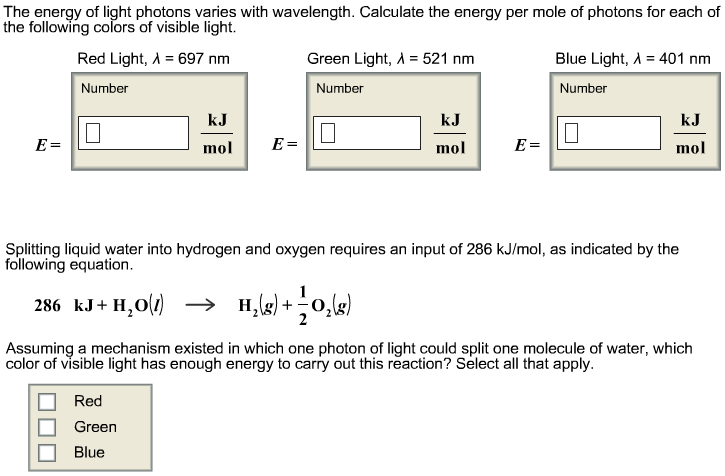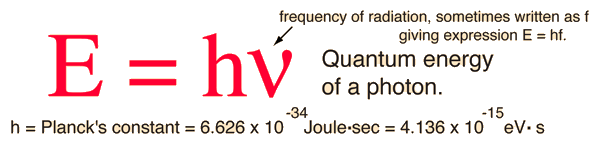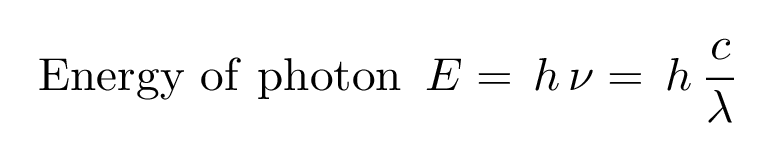
A certain shade of blue has a frequency of 7.32 * 10^14 Hz. What is the energy of exactly one photon of this light? | Socratic
Einstein equation. The equivalent energy E of a body mass M, where C is the speed of light, is the basis for the release of nuclear energy Stock Photo - Alamy

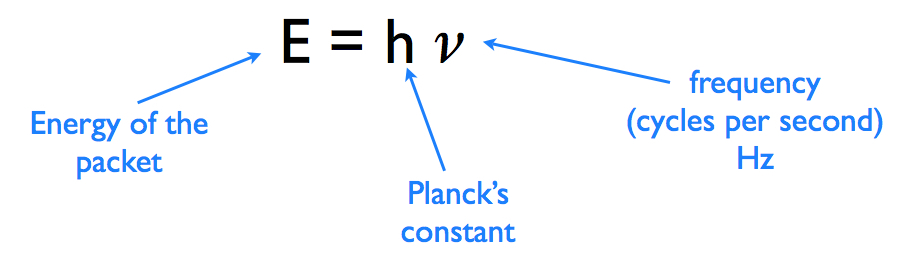
Green light has a frequency of about 6.00 x 10^14 s-1 . What is the energy of a photon of green light? - Home Work Help - Learn CBSE Forum
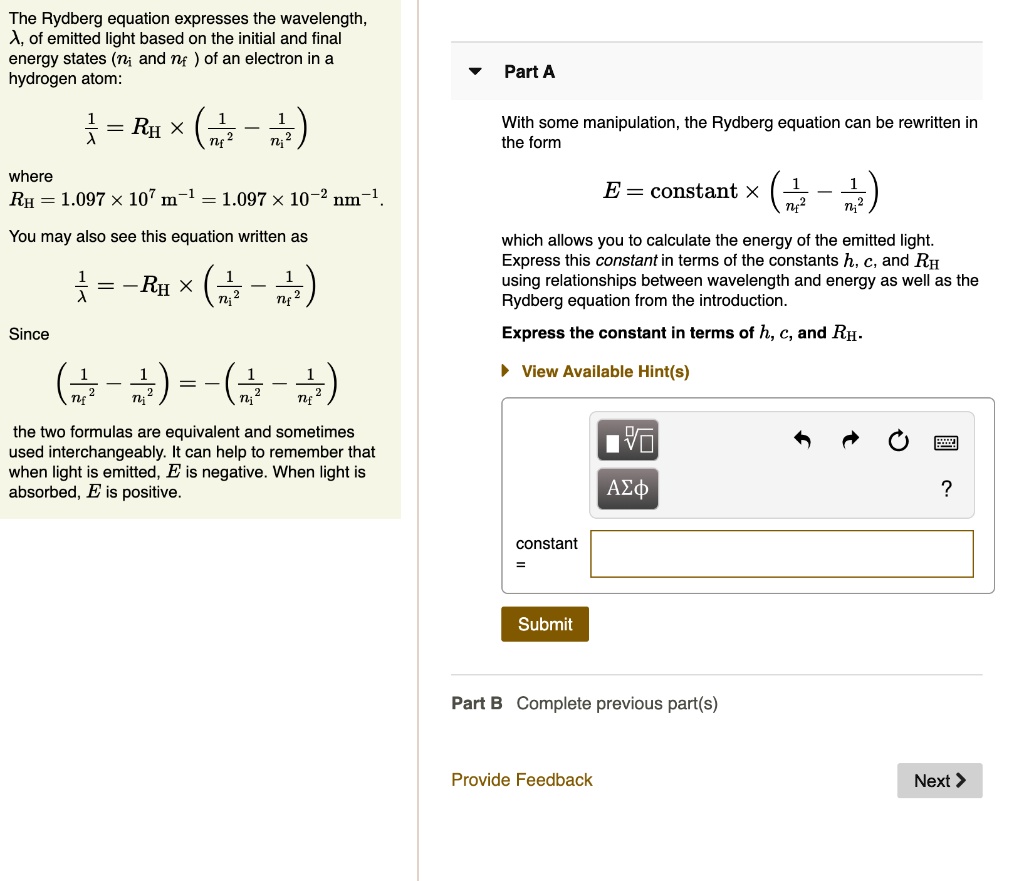
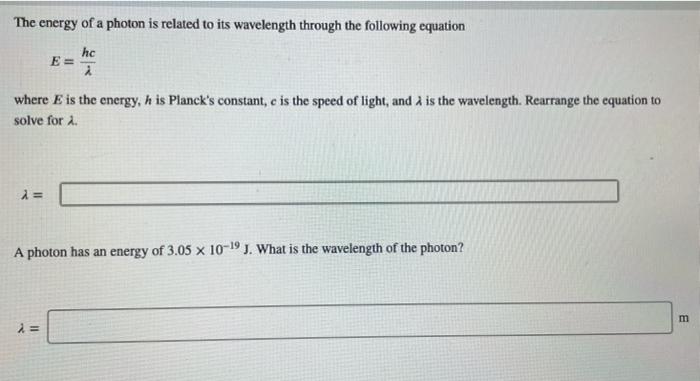
SOLVED:The Rydberg equation expresses the wavelength, A, of emitted light based on the initial and final energy states (ni and nf of an electron in a hydrogen atom: Part A } =